tags:
- monai
- medical
library_name: monai
license: apache-2.0
Model Overview
Body CT segmentation models are evolving. Starting from abdominal multi-organ segmentation model [1]. Now the community is developing hundreds of target anatomies. In this bundle, we provide re-trained models for (3D) segmentation of 104 whole-body segments.
This model is trained using the SegResNet [3] network. The model is trained using TotalSegmentator datasets [2].
Figure source from the TotalSegmentator [2].
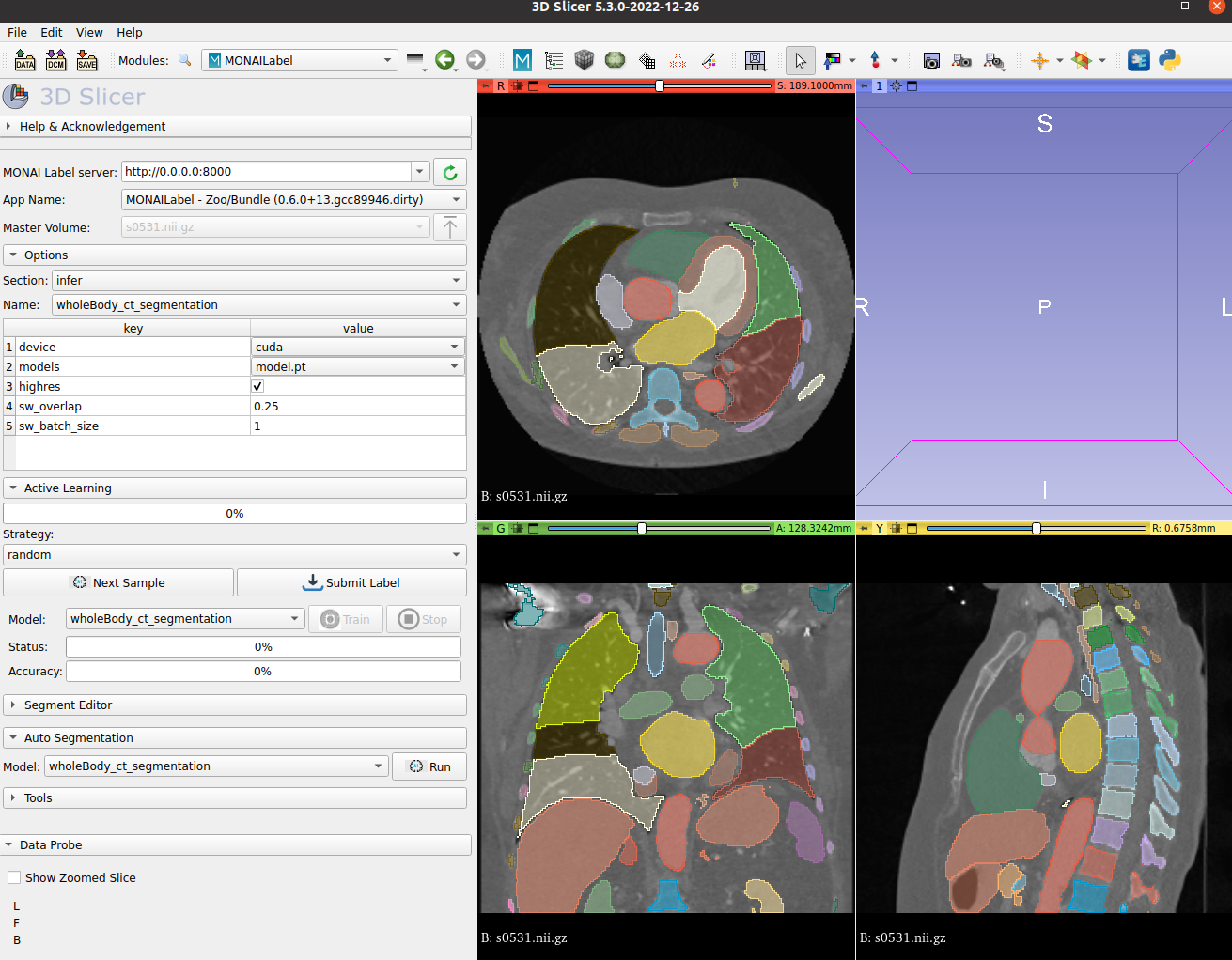
MONAI Label Showcase
- We highlight the use of this bundle to use and visualize in MONAI Label + 3D Slicer integration.
Data
The training set is the 104 whole-body structures from the TotalSegmentator released datasets. Users can find more details on the datasets at https://github.com/wasserth/TotalSegmentator. All rights and licenses are reserved to the original authors.
- Target: 104 structures
- Modality: CT
- Source: TotalSegmentator
- Challenge: Large volumes of structures in CT images
Preprocessing
To use the bundle, users need to download the data and merge all annotated labels into one NIFTI file. Each file contains 0-104 values, each value represents one anatomy class. A sample set is provided with this link.
Training Configuration
The segmentation of 104 tissues is formulated as voxel-wise multi-label segmentation. The model is optimized with the gradient descent method minimizing Dice + cross-entropy loss between the predicted mask and ground truth segmentation.
The training was performed with the following:
- GPU: 32 GB of GPU memory
- Actual Model Input: 96 x 96 x 96
- AMP: True
- Optimizer: AdamW
- Learning Rate: 1e-4
- Loss: DiceCELoss
Input
One channel
- CT image
Output
105 channels
- Label 0: Background (everything else)
- label 1-105: Foreground classes (104)
Resource Requirements and Latency Benchmarks
High-Resolution and Low-Resolution Models
We retrained two versions of the totalSegmentator models, following the original paper and implementation. To meet multiple demands according to computation resources and performance, we provide a 1.5 mm model and a 3.0 mm model, both models are trained with 104 foreground output channels.
In this bundle, we configured a parameter called highres, users can set it to true when using 1.5 mm model, and set it to false to use the 3.0 mm model. The high-resolution model is named model.pt by default, the low-resolution model is named model_lowres.pt.
In MONAI Label use case, users can set the parameter in 3D Slicer plugin to control which model to infer and train.
- Pretrained Checkpoints
- 1.5 mm model: Download link
- 3.0 mm model: Download link
Latencies and memory performance of using the bundle with MONAI Label:
Tested Image Dimension: (512, 512, 397), the slice thickness is 1.5mm in this case. After resample to 1.5 isotropic resolution, the dimension is (287, 287, 397)
1.5 mm (highres) model (Single Model with 104 foreground classes)
Benchmarking on GPU: Memory: 28.73G
++ Latencies => Total: 6.0277; Pre: 1.6228; Inferer: 4.1153; Invert: 0.0000; Post: 0.0897; Write: 0.1995
Benchmarking on CPU: Memory: 26G
++ Latencies => Total: 38.3108; Pre: 1.6643; Inferer: 30.3018; Invert: 0.0000; Post: 6.1656; Write: 0.1786
3.0 mm (lowres) model (single model with 104 foreground classes)
GPU: Memory: 5.89G
++ Latencies => Total: 1.9993; Pre: 1.2363; Inferer: 0.5207; Invert: 0.0000; Post: 0.0358; Write: 0.2060
CPU: Memory: 2.3G
++ Latencies => Total: 6.6138; Pre: 1.3192; Inferer: 3.6746; Invert: 0.0000; Post: 1.4431; Write: 0.1760
Performance
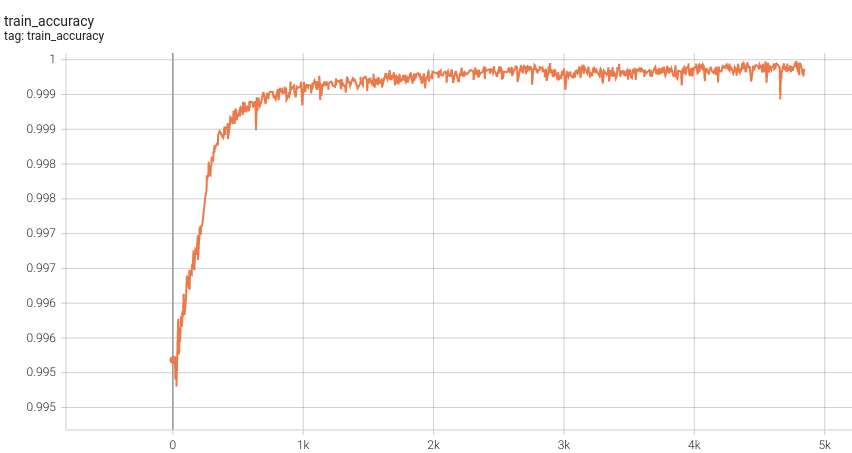
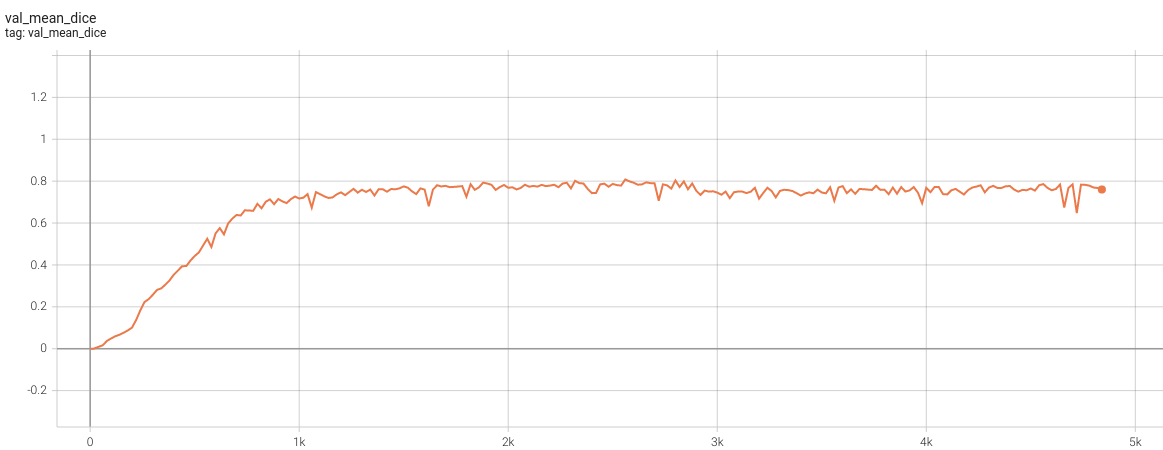
1.5 mm Model Training
Training Accuracy
Validation Dice
Please note that this bundle is non-deterministic because of the trilinear interpolation used in the network. Therefore, reproducing the training process may not get exactly the same performance. Please refer to https://pytorch.org/docs/stable/notes/randomness.html#reproducibility for more details about reproducibility.
MONAI Bundle Commands
In addition to the Pythonic APIs, a few command line interfaces (CLI) are provided to interact with the bundle. The CLI supports flexible use cases, such as overriding configs at runtime and predefining arguments in a file.
For more details usage instructions, visit the MONAI Bundle Configuration Page.
Execute training:
python -m monai.bundle run --config_file configs/train.json
Override the train config to execute multi-GPU training:
torchrun --standalone --nnodes=1 --nproc_per_node=2 -m monai.bundle run --config_file "['configs/train.json','configs/multi_gpu_train.json']"
Please note that the distributed training-related options depend on the actual running environment; thus, users may need to remove --standalone, modify --nnodes, or do some other necessary changes according to the machine used. For more details, please refer to pytorch's official tutorial.
Override the train config to execute evaluation with the trained model:
python -m monai.bundle run --config_file "['configs/train.json','configs/evaluate.json']"
Override the train config and evaluate config to execute multi-GPU evaluation:
torchrun --standalone --nnodes=1 --nproc_per_node=2 -m monai.bundle run --config_file "['configs/train.json','configs/evaluate.json','configs/multi_gpu_evaluate.json']"
Execute inference:
python -m monai.bundle run --config_file configs/inference.json
Execute inference with Data Samples:
python -m monai.bundle run --config_file configs/inference.json --datalist "['sampledata/imagesTr/s0037.nii.gz','sampledata/imagesTr/s0038.nii.gz']"
References
[1] Tang, Y., Gao, R., Lee, H.H., Han, S., Chen, Y., Gao, D., Nath, V., Bermudez, C., Savona, M.R., Abramson, R.G. and Bao, S., 2021. High-resolution 3D abdominal segmentation with random patch network fusion. Medical image analysis, 69, p.101894.
[2] Wasserthal, J., Meyer, M., Breit, H.C., Cyriac, J., Yang, S. and Segeroth, M., 2022. TotalSegmentator: robust segmentation of 104 anatomical structures in CT images. arXiv preprint arXiv:2208.05868.
[3] Myronenko, A., Siddiquee, M.M.R., Yang, D., He, Y. and Xu, D., 2022. Automated head and neck tumor segmentation from 3D PET/CT. arXiv preprint arXiv:2209.10809.
License
Copyright (c) MONAI Consortium
Licensed under the Apache License, Version 2.0 (the "License"); you may not use this file except in compliance with the License. You may obtain a copy of the License at
http://www.apache.org/licenses/LICENSE-2.0
Unless required by applicable law or agreed to in writing, software distributed under the License is distributed on an "AS IS" BASIS, WITHOUT WARRANTIES OR CONDITIONS OF ANY KIND, either express or implied. See the License for the specific language governing permissions and limitations under the License.